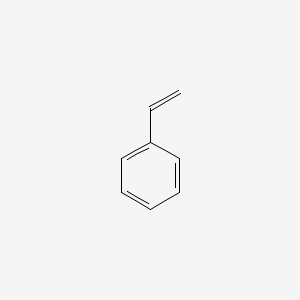
 It's a colorless liquid that evaporates easily and has a sweet smell. It often contains other chemicals that give it a sharp, unpleasant smell. It dissolves in some liquids but doesn't dissolve easily in water.
It's a colorless liquid that evaporates easily and has a sweet smell. It often contains other chemicals that give it a sharp, unpleasant smell. It dissolves in some liquids but doesn't dissolve easily in water. Styrene is an essential component of materials used to make thousands of remarkably strong, flexible, and light-weight products for home, school, work, and play.
Styrene is an essential component of materials used to make thousands of remarkably strong, flexible, and light-weight products for home, school, work, and play.